Recently, Bioer’s HPV Genotyping Real-time PCR Kit (Fluorescent PCR) was approved for marketing by NMPA (National Medical Products Administration). This marks a milestone for Bioer, to enhance reproductive health diagnostics, a new tool has been added to the diagnostic solution.
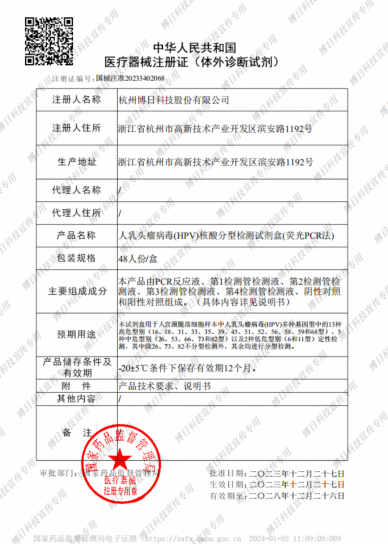
The Bioer HPV Genotyping Real-time PCR Kit (Fluorescent PCR) employs fluorescent quantitative PCR, offering the advantages of high specificity and sensitivity. It not only allows for timely detection in the early stages of virus infection but also differentiates between high-risk and low-risk viral types. This test holds instructive significance for the diagnosis and treatment of HPV.
HPV nucleic acid testing has been incorporated into the cervical cancer screening protocols of multiple countries.
Human Papillomavirus (HPV) is an enveloped, double-stranded, circular DNA virus with an affinity for epithelial tissues. Based on its potential to induce cancer, it is categorized into high-risk and low-risk types. It is now well-established that persistent infection with high-risk HPV is a key factor in the development of cervical cancer and precancerous lesions.
In China, the "Guidelines for HPV Nucleic Acid Detection, Genotyping, and Reagent Technology Review" released in 2015 classifies 13 genotypes, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, as high-risk HPV (HR-HPV).
The 2021 WHO Second Edition of the "Guidelines for the Prevention of Cervical Cancer: Screening and Treatment of Precancerous Lesions" recommends HPV DNA testing as the preferred method for cervical cancer screening.
The expert consensus "Application of HPV DNA Testing in the Initial Screening of Cervical Cancer in Health Examination Populations (2022)," "Chinese Expert Consensus on HPV Nucleic Acid Detection for Cervical Cancer Screening (2022)," and "Chinese Guidelines for Cervical Cancer Screening (2023)" all recommend high-risk HPV nucleic acid testing as the primary screening method.
Reproductive health is a focal point in Bioer's infectious disease testing endeavors, and the introduction of the HPV 20 genotyping further enhances Bioer's product portfolio in reproductive infection detection. Bioer Technology offers a comprehensive solution encompassing nucleic acid testing instruments, reagents, and consumables. The instrument platform covers a wide range of solutions, including fully automated nucleic acid extraction, sample partitioning platforms, fluorescent quantitative PCR instruments, and all-in-one systems for automated nucleic acid extraction and testing. These solutions cater to laboratories of varying scales, providing multidimensional product services for small, medium, and large-scale molecular testing experiments.

